IN THE BEGINNING…
With the passage of the United States Clean Air Act in 1970, auto manufacturers were faced with the task of substantially reducing emissions. The catalytic converter played a big role in manufacturers meeting the new standard. Check out how the design has changed over the years with the transition from the two-way design to the modern three-way converters in use today.
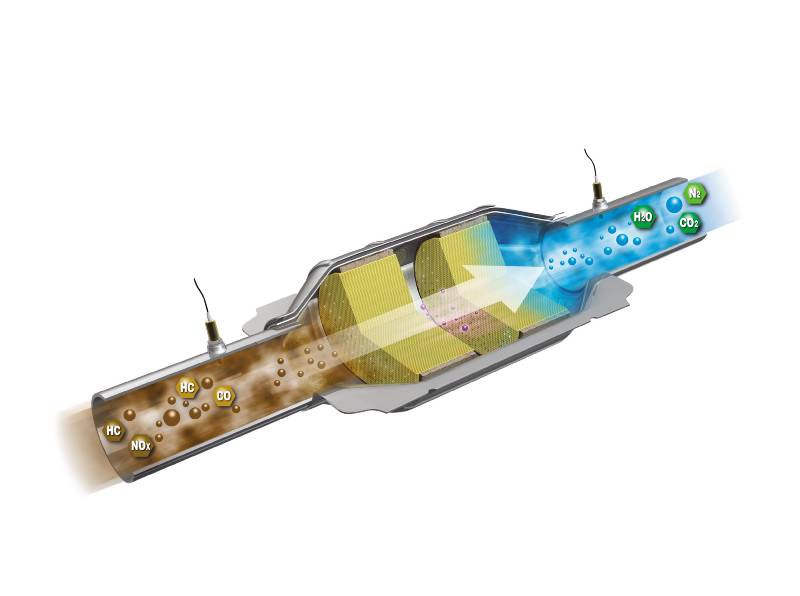
Two-Way Catalytic Converters
Allows oxidation of CO (carbon monoxide) to less-harmful CO2 (carbon dioxide)
Allows oxidation of HC (unburned hydrocarbons) to CO2 (carbon dioxide) and H2O (water)
In this design, exhaust gases are directed to flow through the substrate, containing precious metals platinum and palladium, which allow the chemical reaction to occur. The exhaust gases increase in temperature as the conversion process takes place.
Because of the intense heat created by this process, exhaust gases leaving the converter should be hotter than the gases entering the converter. This also explains why heat shields are required on most units.
Two-way converters operate relatively efficiently with a lean fuel mixture. Ineffectiveness in controlling NOx (nitrogen oxides) led to the introduction of three-way converters.
Three-Way Plus Air Catalytic Converters
Allows reduction of NOx (nitrogen oxides) to N2 (nitrogen) and O2 (oxygen)
Allows oxidation of CO (carbon monoxide) to less-harmful CO2 (carbon dioxide)
Allows oxidation of HC (unburned hydrocarbons) to CO2 (carbon dioxide) and H2O (water)
Three-way plus air converters were used in vehicle emissions systems in North America during the late '70s and early '80s.
Inside this converter there are two substrates. The front, coated with the precious metal rhodium, is used to reduce NOx emissions into simple N2 and O2. This process is most effective when little O2 is present (rich mixture). That is why it is located upstream of the air tube.
Since a rich mixture is high in HC and CO, an air pump and tube supply additional O2 to this mixture before it enters the second substrate.
The second substrate, coated with the precious metals palladium and platinum, allows oxidation of HC and CO to less harmful emissions CO2 and H2O.
This system was not very efficient and was phased out in the early '80s, when the current three-way converter was introduced.
Three-Way Catalytic Converters
Allows reduction of NOx (nitrogen oxides) to N2 (nitrogen) and O2 (oxygen)
Allows oxidation of CO (carbon monoxide) to less-harmful CO2 (carbon dioxide)
Allows oxidation of HC (unburned hydrocarbons) to CO2 (carbon dioxide) and H2O (water)
Three-way converters have been used in vehicle emissions control systems in North America - and many other countries - since 1981.
The three-way converter without air uses advanced catalyst chemistry to store and release O2, in conjunction with an O2 monitoring and control system.
This system utilizes one or more O2 sensors to oscillate the fuel mixture between lean and rich conditions. This oscillation, combined with the O2 storage and release on the catalyst surface, allows for optimum reduction of all three emissions.
Three-way converters are used in conjunction with OBDII diagnostic systems on today's vehicles. This system alerts the driver when the converter is not working at peak efficiency.
Learn more about quality exhaust parts, find the right car part, or find a local repair shop today.
The content contained in this article is for informational purposes only and should not be used in lieu of seeking professional advice from a certified technician or mechanic. We encourage you to consult with a certified technician or mechanic if you have specific questions or concerns relating to any of the topics covered herein. Under no circumstances will we be liable for any loss or damage caused by your reliance on any content.