Catalytic Converter Facts
Catalytic converters have been part of the exhaust system of vehicles equipped with an internal combustion engine since their mandated use in the mid-1970s. Designed to convert dangerous gases into less toxic emissions, some modern catalytic converters may also play a role in ensuring the efficiency of the engine.

Catalytic Converter Basics
Designed for long life
Catalytic converters should last the life of the vehicle if operating conditions are correct.
Don’t fail on their own
There is usually an underlying issue if a catalytic converter fails. If you find yourself repeatedly replacing a catalytic converter on a vehicle, it’s time to do some investigation. Before installing another converter on the vehicle, run through these five steps to take before replacing a catalytic converter.
Stores oxygen
There are no moving parts in a catalytic converter, it mainly stores oxygen and releases it when necessary to reduce emissions. Emissions aren’t eliminated rather they’re converted into less toxic substances through chemical reactions in the converter.
Design
There are three main ways converters are designed. Older converters were pelletized, which contributed to the thought that converters were restrictive and hurt performance and fuel efficiency. However, current converters are designed with one or more steel or ceramic substrates depending on the emission system and durability requirements. Steel substrate converters are far less efficient than ceramic substrates, however they’re more resistant to failure due to cold quenching which is why they are mainly used in some off-road applications. Both steel and ceramic substrates have very low restriction and when sized properly never cause a loss in power or efficiency.

Catalytic Converter Construction, Operation & Chemistry
For a catalytic converter to work it requires two things:
Heat
A result of the combustion process, many modern converters are closed couple which means they are mounted within a foot of the engine.
Perfect balance of air and fuel
This is tightly controlled by modern fuel management systems which may use oxygen sensors, mass air flow sensors and MAP sensors, along with input from many other sensors to achieve the perfect balance of air and fuel
How Does a Catalytic Converter Store Oxygen?
The converter’s ceramic substrate is covered in a washcoat that contains precious metals such as rhodium, platinum, palladium, cerium and nickel. As dangerous gases come into the converter, they bond to the precious metals. As dangerous emissions such as hydrocarbons and CO pass through the converter, the converter releases stored oxygen which allows a chemical reaction to convert the harmful emissions into CO2 and water. This reaction produces heat to help maintain the converter temperature and keep the substrate clean. In addition, the converter also allows any nitrogen that may have combined with oxygen to form NOx to separate and become free nitrogen once again. The oxygen released is stored on the converter substrate to allow this process to happen continuously.
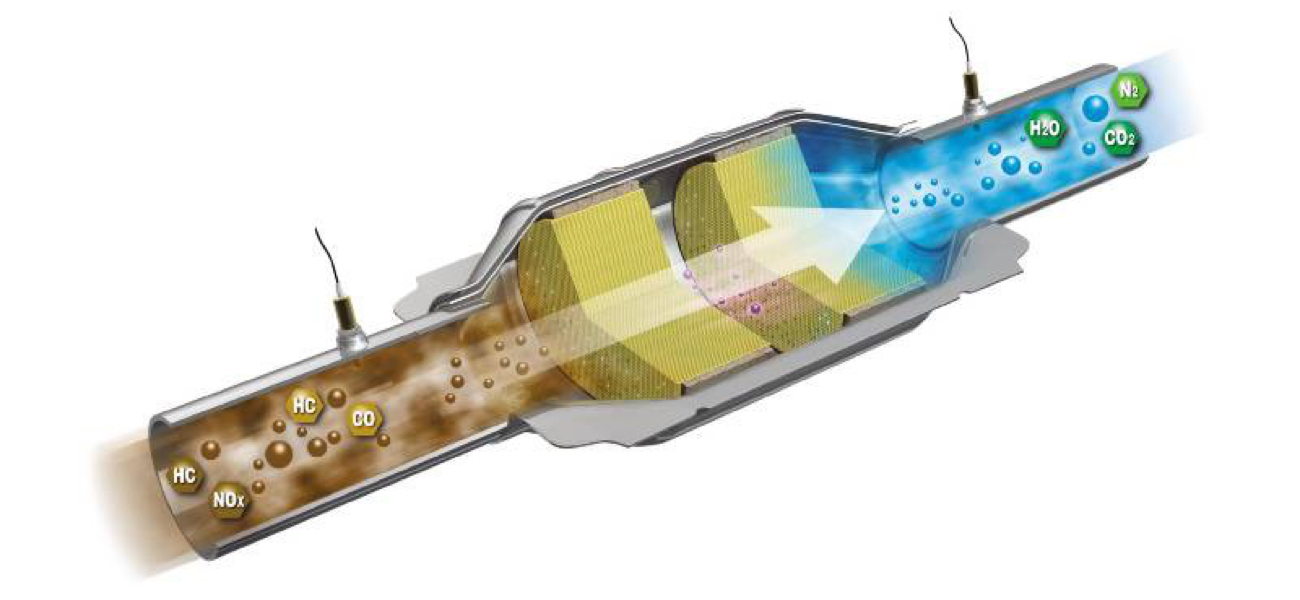
Harmful Emissions (Product of Incomplete Combustion) |
||
|---|---|---|
| HC | Hydrocarbon | Unburnt Fuel |
| CO | Carbon Monoxide | Partially burnt fuel or oil |
| NOx | Oxides of Nitrogen | Extreme combustion pressure / temperature |
Resulting Emissions (Byproduct of Complete Combustion) |
|
|---|---|
| H20 | Water |
| CO2 | Carbon Dioxide |
| N2 | Nitrogen |
Engine Air/Fuel Ratio Operating Range versus Converter Air/Fuel Ratio Operating Range
Due to the advantages of compression and spark, an engine can operate under a wide range of air/fuel ratios. However, a converter needs the air/fuel ratio balance to be perfect or its efficiency will drop rapidly.
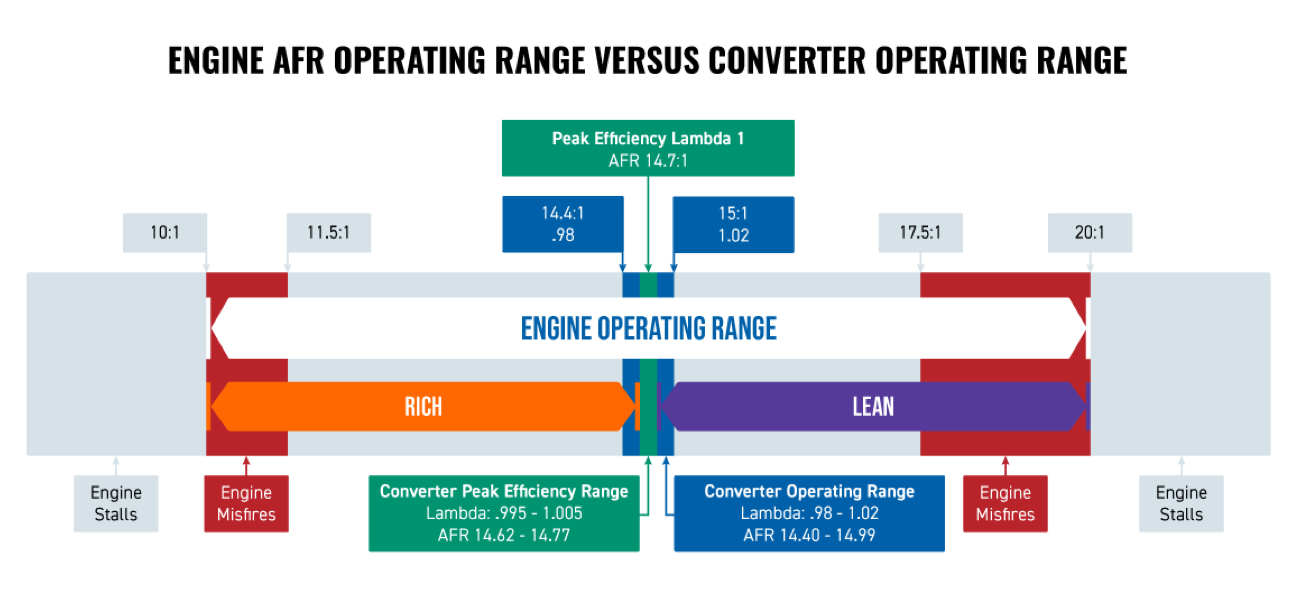
As depicted in the chart above, the air/fuel ratio operating range of the converter is extremely tight as compared to an engine. The efficiency of a converter drops much more rapidly than an engine will when the air/fuel ratio moves away from the perfect Stoichiometric ratio (also known as Lambda).
Exhaust leaks are one of the main reasons why the converter feed gases air/fuel ratios are not always perfect. However, there are a multitude of other issues which can cause this problem such as a leaking or restrictive fuel injector, intake manifold leaks, and defective O2 sensors, mass air flow sensors, MAP sensors, etc.
Why Catalytic Converter Fails
These conditions are NOT warrantable:

Remember, catalytic converters don’t fail on their own, it is important to identify the root cause of the failure and perform the repair(s) prior to installing a new converter.
Overheated/Melted Substrate
As mentioned earlier, the chemical reaction happening in the converter generates heat. If the converter is asked to do more work than it was designed to, it may reach temperatures high enough to melt the ceramic substrate. This is typically the result of an ignition misfire or low compression in a cylinder.
Oil Coated or Fouled Substrate
Oil leaking by the valve seals or piston rings can get into the combustion chamber where it doesn’t burn off and gets into the converter. If the washcoat gets soaked in oil, the harmful gases will no longer bond to the precious metals. An internal coolant leak will cause the same problems for the washcoat.
Structural Damage
Something has impacted the converter and caused structural damage. The substrate comes loose making it unable to perform.
Learn more about quality exhaust parts, find the right car part, or find a local repair shop today.
The content in this article is for informational purposes only. You should consult with a certified technician or mechanic if you have questions relating to any of the topics covered herein. Tenneco will not be liable for any loss or damage caused by your reliance on any content.